


The international chemical industry is still largely based on fossil raw materials and is the industrial sector with the highest energy consumption worldwide (IEA). For this reason, it is important to investigate alternative synthesis processes that are based on renewable raw materials and are more energy-efficient. One such process is the oxidative dehydration of (bio)ethanol to acetaldehyde. Acetaldehyde is a so-called chemical “feedstock”, i.e. an intermediate product of the chemical industry, of which around 1.5 million tons are produced annually. And the trend is rising. Acetaldehyde is currently obtained from ethylene (a product of natural gas steam cracking) in the Wacker Höchst process. The process uses an expensive and toxic palladium chloride catalyst. The oxidative dehydration of (bio)ethanol therefore has two major advantages: it uses a renewable raw material and the catalyst, iron oxide with a low molybdenum content, is inexpensive and not harmful to the environment.
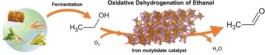
Process illustration of the oxidative dehydrogenation of bioethanol to acetaldehyde. Source: doi.org/10.1002/cctc.202101219
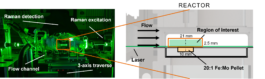
Photo of the catalysis channel with the green laser of the Dual-Track Raman spectrometer. Right: Sectional view showing the region of interest of the measurement in the reactor of the catalysis channel above the catalyst pellet.
Before novel synthesis routecan be implemented in the chemical industry, they must be examined for various properties. The coupling of the flow in the gas phase (mass and heat transport) with the reaction on the surface plays an important role here. Using spontaneous Raman spectroscopy, we measure gas concentrations and temperatures in the gas phase non-intrusively and while the process is taking place (operando). This allows us to gain important insights into the characteristics of the catalyst, but also into optimal operating conditions.
For this purpose, we have developed a new type of Raman spectrometer, the DTRS, which allows us to measure the two parameters (concentrations and temperature) with high spatial resolution and in close proximity to the catalyst surface. The catalyst itself is integrated into an optically accessible and temperature-controlled flow channel, which was developed in student work.
Using IR thermography, we can also observe the temperature of the catalyst surface with a thermal imaging camera and thus detect inhomogeneities and hotspots.
This experimental information is compared with a CFD simulation (digital twin) in order to predict the behavior of the system under different conditions.
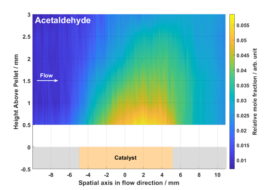
Two-dimensional plot of the measured relative concentration of the product species acetaldehyde using Raman spectroscopy.
With our activities, we are part of the Collaborative Research Center 1487 “Iron, upgraded!” of the German Research Foundation. As part of the first funding period from 2022 to 2026, the use of iron-based materials is being investigated with a focus on their magnetic and catalytic properties. Iron is the most abundant metal in the earth's crust and could replace rare, toxic and environmentally harmful metals in essential chemical applications in the future. In this project, a consortium led by TU Darmstadt in collaboration with Goethe University Frankfurt, Heidelberg University, Johannes Gutenberg University Mainz, Philipps University Marburg and the Max Planck Institute for Chemical Energy Conversion will comprehensively investigate iron in various environments and with regard to a wide range of parameters. The aim is to come to meaningful conclusions about the future use of iron and to develop solutions for sustainable processes and materials.
Further information can be found here: https://www.chemie.tu-darmstadt.de/iron-upgraded/
